CAVITATION AND BUBBLE DYNAMICS
by Christopher Earls Brennen © Oxford University Press 1995
CHAPTER 2.
SPHERICAL BUBBLE DYNAMICS
2.1 INTRODUCTION
Having considered the initial formation of bubbles, we now proceed to identify the subsequent dynamics of bubble growth and collapse. The behavior of a single bubble in an infinite domain of liquid at rest far from the bubble and with uniform temperature far from the bubble will be examined first. This spherically symmetric situation provides a simple case that is amenable to analysis and reveals a number of important phenomena. Complications such as those introduced by the presence of nearby solid boundaries will be discussed in the chapters which follow.
Consider a spherical bubble of radius, R(t) (where t is time), in an infinite domain of liquid whose temperature and pressure far from the bubble are T∞ and p∞(t) respectively. The temperature, T∞ , is assumed to be a simple constant since temperature gradients were eliminated a priori and uniform heating of the liquid due to internal heat sources or radiation will not be considered. On the other hand, the pressure, p∞(t), is assumed to be a known (and perhaps controlled) input which regulates the growth or collapse of the bubble.
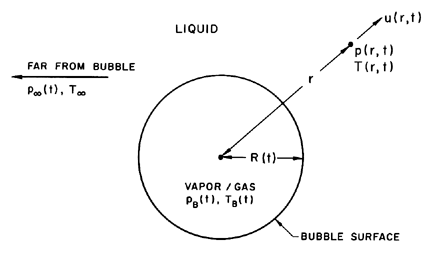
|
| Figure 2.1 Schematic of a spherical bubble in an infinite liquid. |
Though compressibility of the liquid can be important in the context of bubble collapse, it will, for the present, be assumed that the liquid density, ρL , is a constant. Furthermore, the dynamic viscosity, μL, is assumed constant and uniform. It will also be assumed that the contents of the bubble are homogeneous and that the temperature, TB(t), and pressure, pB(t), within the bubble are always uniform. These assumptions may not be justified in circumstances that will be identified as the analysis proceeds.
The radius of the bubble, R(t), will be one of the primary results of the analysis. As indicated in Figure 2.1, radial position within the liquid will be denoted by the distance, r, from the center of the bubble; the pressure, p(r,t) , radial outward velocity, u(r,t), and temperature, T(r,t), within the liquid will be so designated. Conservation of mass requires that
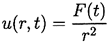 | ......(2.1) |
|---|
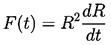 | ......(2.2) |
|---|
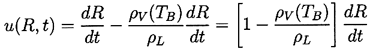 | ......(2.3) |
|---|
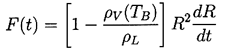 | ......(2.4) |
|---|
Assuming a Newtonian liquid, the Navier-Stokes equation for motion in the r direction,
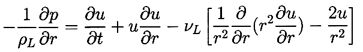 | ......(2.5) |
|---|
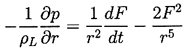 | ......(2.6) |
|---|
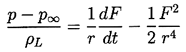 | ......(2.7) |
|---|
|
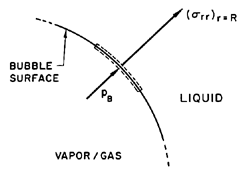
| Figure 2.2 Portion of the spherical bubble surface. |
To complete this part of the analysis, a dynamic boundary condition on the bubble surface must be constructed. For this purpose consider a control volume consisting of a small, infinitely thin lamina containing a segment of interface (Figure 2.2). The net force on this lamina in the radially outward direction per unit area is
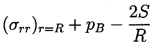 | ......(2.8) |
|---|
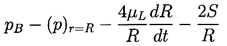 | ......(2.9) |
|---|
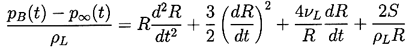 | ......(2.10) |
|---|
2.3 BUBBLE CONTENTS
In addition to the Rayleigh-Plesset equation, considerations of the bubble contents are necessary. To be fairly general, it is assumed that the bubble contains some quantity of contaminant gas whose partial pressure is pGo at some reference size, Ro, and temperature, T∞ . Then, if there is no appreciable mass transfer of gas to or from the liquid, it follows that
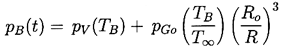 | ......(2.11) |
|---|
It remains to determine TB(t). This is not always necessary since, under some conditions, the difference between the unknown TB and the known T∞ is negligible. But there are also circumstances in which the temperature difference, (TB(t)-T∞), is important and the effects caused by this difference dominate the bubble dynamics. Clearly the temperature difference, (TB(t)-T∞), leads to a different vapor pressure, pV(TB), than would occur in the absence of such thermal effects, and this alters the growth or collapse rate of the bubble. It is therefore instructive to substitute Equation 2.11 into 2.10 and thereby write the Rayleigh-Plesset equation in the following general form:
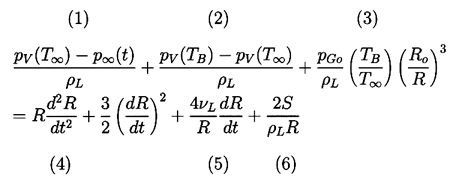 | ......(2.12) |
|---|
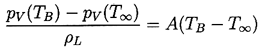 | ......(2.13) |
|---|
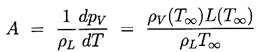 | ......(2.14) |
|---|
The degree to which the bubble temperature, TB, departs from the remote liquid temperature, T∞, can have a major effect on the bubble dynamics, and it is neccessary to discuss how this departure might be evaluated. The determination of (TB-T∞) requires two steps. First, it requires the solution of the heat diffusion equation,
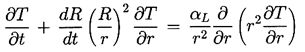 | ......(2.15) |
|---|
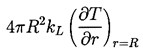 | ......(2.16) |
|---|
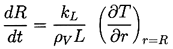 | ......(2.17) |
|---|
The nature of the thermal effect problem is now clear. The thermal term in the Rayleigh-Plesset Equation 2.12 requires a relation between (TB(t)-T∞) and R(t). The energy balance Equation 2.17 yields a relation between (∂T/∂r)r=R and R(t). The final relation between (∂T/∂r)r=R and (TB(t)-T∞) requires the solution of the heat diffusion equation. It is this last step that causes considerable difficulty due to the evident nonlinearities in the heat diffusion equation; no exact analytic solution exists. However, the solution of Plesset and Zwick (1952) provides a useful approximation for many purposes. This solution is confined to cases in which the thickness of the thermal boundary layer, δT, surrounding the bubble is small compared with the radius of the bubble, a restriction that can be roughly represented by the identity
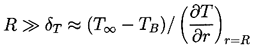 | ......(2.18) |
|---|
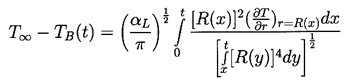 | ......(2.19) |
|---|
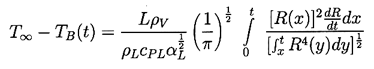 | ......(2.20) |
|---|
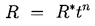 | ......(2.21) |
|---|
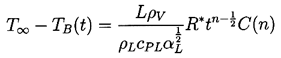 | ......(2.22) |
|---|
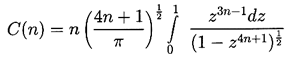 | ......(2.23) |
|---|
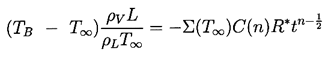 | ......(2.24) |
|---|
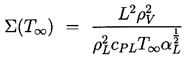 | ......(2.25) |
|---|
2.4 IN THE ABSENCE OF THERMAL EFFECTS
First we consider some of the characteristics of bubble dynamics in the absence of any significant thermal effects. This kind of bubble dynamic behavior is termed ``inertially controlled'' to distinguish it from the ``thermally controlled'' behavior discussed later. Under these circumstances the temperature in the liquid is assumed uniform and term (2) in the Rayleigh-Plesset Equation 2.12 is zero.
Furthermore, it will be assumed that the behavior of the gas in the bubble is polytropic so that
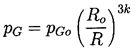 | ......(2.26) |
|---|
With the above assumptions the Rayleigh-Plesset equation becomes
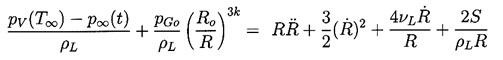 | ......(2.27) |
|---|
|
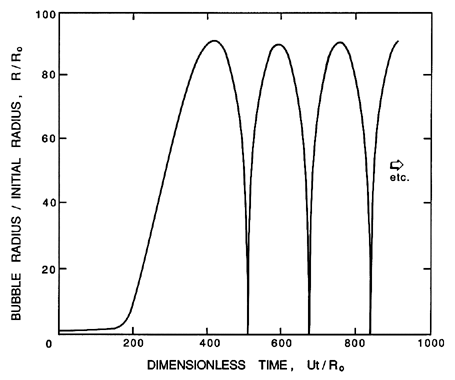
| Figure 2.3 Typical solution of the Rayleigh-Plesset equation for spherical bubble size/ initial size, R/R0. The nucleus enters a low-pressure region at a dimensionless time of 0 and is convected back to the original pressure at a dimensionless time of 500. The low-pressure region is sinusoidal and symmetric about 250. |
Equation 2.27 can be readily integrated numerically to find R(t) given the input p∞(t), the temperature T∞, and the other constants. Initial conditions are also required and, in the context of cavitating flows, it is appropriate to assume that the microbubble of radius Ro is in equilibrium at t=0 in the fluid at a pressure p∞(0) so that
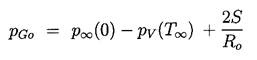 | ......(2.28) |
|---|
Analytic solutions to Equation 2.27 are limited to the case of a step function change in p∞. Nevertheless, these solutions reveal some of the characteristics of more general pressure histories, p∞(t), and are therefore valuable to document. Denoting the constant value of p∞(t>0) by p∞*, Equation 2.27 can be integrated by multiplying through by 2R2dR/dt and forming time derivatives. Only the viscous term cannot be integrated in this way, and what follows is confined to the inviscid case. After integration, application of the initial condition (dR/dt)t=0=0 yields
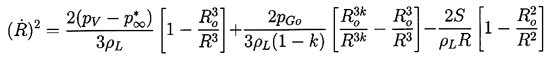 | ......(2.29) |
|---|
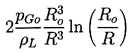 | ......(2.30) |
|---|
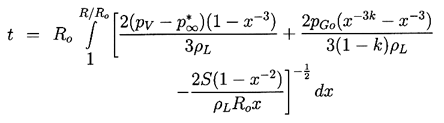 | ......(2.31) |
|---|
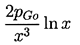 | ......(2.32) |
|---|
Consider first the characteristic behavior for bubble growth which this solution exhibits when p∞*<p∞(0). Equation 2.29 shows that the asymptotic growth rate for R»Ro is given by
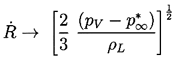 | ......(2.33) |
|---|
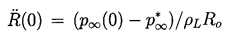 | ......(2.34) |
|---|
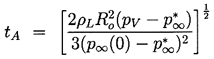 | ......(2.35) |
|---|
Now contrast the behavior of a bubble caused to collapse by an increase in p∞ to p*∞. In this case when R«Ro Equation 2.29 yields
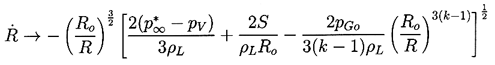 | ......(2.36) |
|---|
For a bubble with a substantial gas content the asymptotic collapse velocity given by Equation 2.36 will not be reached and the bubble will simply oscillate about a new, but smaller, equilibrium radius. On the other hand, when the bubble contains very little gas, the inward velocity will continually increase (like R-3/2) until the last term within the square brackets reaches a magnitude comparable with the other terms. The collapse velocity will then decrease and a minimum size given by
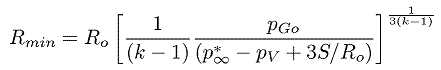 | ......(2.37) |
|---|
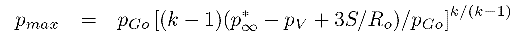 | ......(2.38) |
|---|
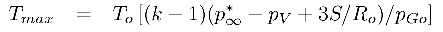 | ......(2.39) |
|---|
The case of zero gas content presents a special albeit somewhat hypothetical problem, since apparently the bubble will reach zero size and at that time have an infinite inward velocity. In the absence of both surface tension and gas content, Rayleigh (1917) was able to integrate Equation 2.31 to obtain the time, tTC, required for total collapse from R=Ro to R=0:
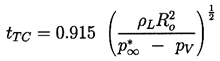 | ......(2.40) |
|---|
It is important at this point to emphasize that while the above results for bubble growth are quite practical, the results for bubble collapse may be quite misleading. Apart from the neglect of thermal effects, the analysis was based on two other assumptions that may be violated during collapse. Later we shall see that the final stages of collapse may involve such high velocities (and pressures) that the assumption of liquid incompressibility is no longer appropriate. But, perhaps more important, it transpires (see Chapter 5) that a collapsing bubble loses its spherical symmetry in ways that can have important engineering consequences.
2.5 STABILITY OF VAPOR/GAS BUBBLES
Apart from the characteristic bubble growth and collapse processes discussed in the last section, it is also important to recognize that the equilibrium condition
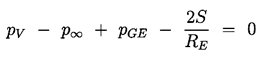 | ......(2.41) |
|---|
Consider a small perturbation in the size of the bubble from R=RE to R=RE(1+ε), ε«1 and the response resulting from the Rayleigh-Plesset equation. Care must be taken to distinguish two possible cases:
- The partial pressure of the gas remains the same at pGE.
- The mass of gas in the bubble and its temperature, TB, remain the same.
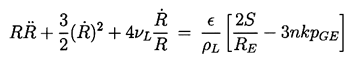 | ......(2.42) |
|---|
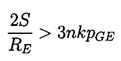 | ......(2.43) |
|---|
First consider Case (i) which must always be unstable since the inequality 2.43 always holds if n=0. This is simply a restatement of the fact (discussed in Section 2.6) that, if one allows time for mass diffusion, then all bubbles will either grow or shrink indefinitely.
Case (ii) is more interesting since in many of the practical engineering situations pressure levels change over a period of time that is short compared with the time required for significant gas diffusion. In this case a bubble in stable equilibrium requires
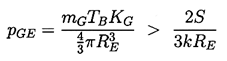 | ......(2.44) |
|---|
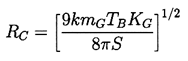 | ......(2.45) |
|---|
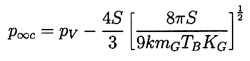 | ......(2.46) |
|---|
|
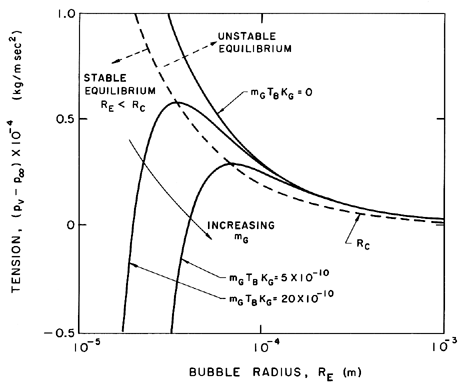
| Figure 2.4 Stable and unstable bubble equilibrium radii as a function of the tension for various masses of gas in the bubble. Stable and unstable conditions are separated by the dotted line. Adapted from Daily and Johnson (1956). |
The isothermal case (k=1) is presented graphically in Figure 2.4 where the solid lines represent equilibrium conditions for a bubble of size RE plotted against the tension (pV -p∞) for various fixed masses of gas in the bubble and a fixed surface tension. The critical radius for any particular mG corresponds to the maximum in each curve. The locus of the peaks is the graph of RC values and is shown by the dashed line whose equation is (pV -p∞)=4S/3RE. The region to the right of the dashed line represents unstable equilibrium conditions. This graphical representation was used by Daily and Johnson (1956) and is useful in visualizing the quasistatic response of a bubble when subjected to a decreasing pressure. Starting in the fourth quadrant under conditions in which the ambient pressure p∞>pV, and assuming the mass of gas in the bubble is constant, the radius RE will first increase as (pV -p∞) increases. The bubble will pass through a series of stable equilibrium states until the particular critical pressure corresponding to the maximum is reached. Any slight decrease in p∞ below the value corresponding to this point will result in explosive cavitation growth regardless of whether p∞ is further decreased or not. Indeed, it is clear from this analysis that the critical tension for a liquid should be given by 4S/3R rather than 2S/R as maintained in Chapter 1, since stable equilibrium conditions do not exist in the range
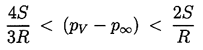 | ......(2.47) |
|---|
This stability phenomenon has important consequences in many cavitating flows. To recognize this, one must visualize a spectrum of sizes of cavitation nuclei being convected into a region of low pressure within the flow. Then the p∞ in Equations 2.41 and 2.47 will be the local pressure in the liquid surrounding the bubble, and p∞ must be less than pV for explosive cavitation growth to occur. It is clear from the above analysis that all of the nuclei whose size, R, is greater than some critical value will become unstable, grow explosively, and cavitate, whereas those nuclei smaller than that critical size will react passively and will therefore not become visible to the eye. Though the actual response of the bubble is dynamic and p∞ is changing continuously, we can nevertheless anticipate that the crtical nuclei size will be given approximately by 4S/3(pV -p∞)* where (pV -p∞)* is some representative measure of the tension in the low-pressure region. Note that the lower the pressure level, p∞, the smaller the critical size and the larger the number of nuclei that are activated. This accounts for the increase in the number of bubbles observed in a cavitating flow as the pressure is reduced.
|
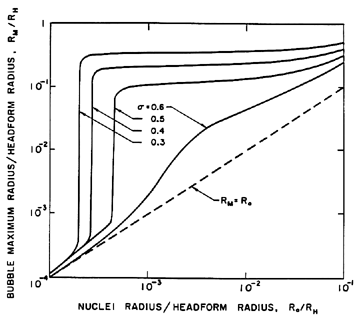
| Figure 2.5 The maximum size, RM , to which a cavitation bubble grows according to the Rayleigh-Plesset equation as a function of the original nucleus size, Ro, and the cavitation number, σ, in the flow around an axisymmetric headform of radius, RH , with Weber number, ρLRHU∞2/S=28000 (from Ceccio and Brennen 1991). |
A quantitative example of this effect is shown in Figure 2.5, which presents results from the integration of the Rayleigh-Plesset equation for bubbles in the flow around an axisymmetric headform. It shows the maximum size which the bubbles achieve as a function of the size of the original nucleus for a typical Weber number, ρLRHU∞2/S, of 28000 where U∞ and RH are the free stream velocity and headform radius. Data are plotted for four different cavitation numbers, σ, representing different ambient pressure levels. Note that the curves for σ<0.5 all have abrupt vertical sections at certain critical nuclei sizes and that this critical size decreases with decreasing σ. Numerical results for this and other flows show that the critical size, RC, adheres fairly closely to the nondimensional version of the expression derived earlier,
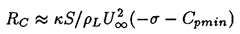 | ......(2.48) |
|---|
Note also from Figure 2.5 that, whatever their initial size, all unstable nuclei grow to roughly the same maximum size. This is because both the asymptotic growth rate and the time available for growth are relatively independent of the size of the original nucleus. From Equation 2.33 the growth rate is given approximately by
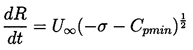 | ......(2.49) |
|---|
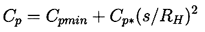 | ......(2.50) |
|---|
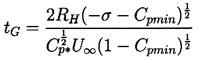 | ......(2.51) |
|---|
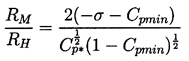 | ......(2.52) |
|---|
In most of the circumstances considered in this chapter, it is assumed that the events occur too rapidly for significant mass transfer of contaminant gas to occur between the bubble and the liquid. Thus we assumed in Section 2.3 and elsewhere that the mass of contaminant gas in the bubble remained constant. It is convenient to reconsider this issue at this point, for the methods of analysis of mass diffusion will clearly be similar to those of thermal diffusion (Scriven 1959). Moreover, there are some issues that require analysis of the rate of increase or decrease of the mass of gas in the bubble. One of the most basic issues is the fact that any and all of the gas-filled microbubbles that are present in a subsaturated liquid (and particularly in water) should dissolve away if the ambient pressure is sufficiently high. Henry's law states that the partial pressure of gas, pGE, in the bubble, which is in equilibrium with a saturated concentration, c∞, of gas dissolved in the liquid will be given by
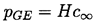 | ......(2.53) |
|---|
The process of mass transfer can be analysed by noting that the concentration, c(r,t), of gas in the liquid will be governed by a diffusion equation identical in form to Equation 2.15,
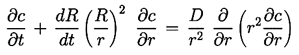 | ......(2.54) |
|---|
The simplest problem is that of a bubble of radius, R, in a liquid at a fixed ambient pressure, p∞, and gas concentration, c∞. In the absence of inertial effects the partial pressure of gas in the bubble will be pGE where
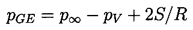 | ......(2.55) |
|---|
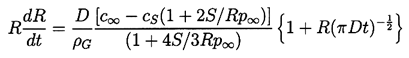 | ......(2.56) |
|---|
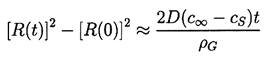 | ......(2.57) |
|---|
It is instructive to evaluate the typical duration of growth (or shrinkage). From Equation 2.57 the time required for complete solution is tCS where
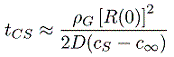 | ......(2.58) |
|---|
The fact that a microbubble should dissolve within seconds leaves unresolved the question of why cavitation nuclei persist indefinitely. One possible explanation is that the interface is immobilized by the effects of surface contamination. Another is that the bubble is imbedded in a solid particle in a way that inhibits the solution of the gas, the so-called Harvey nucleus. These issues were discussed previously in Section 1.12.
Finally we note that there is an important mass diffusion effect caused by ambient pressure oscillations in which nonlinearities can lead to bubble growth even in a subsaturated liquid. This is known as ``rectified diffusion'' and is discussed later in Section 4.9.
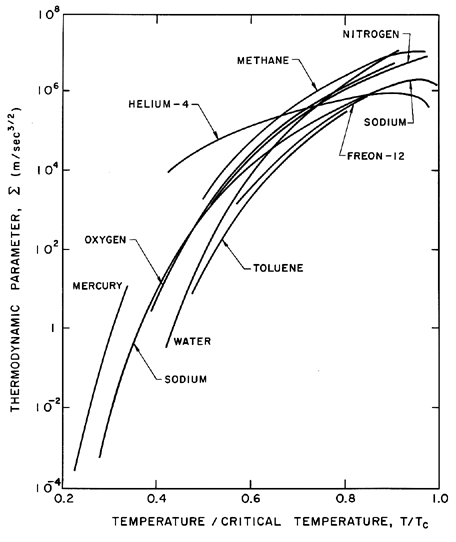
In Sections 2.4 through 2.6 some of the characteristics of bubble dynamics in the absence of thermal effects were explored. It is now necessary to examine the regime of validity of these analyses, and it is convenient to first evaluate the magnitude of the thermal term 2.24 which was neglected in Equation 2.12 in order to produce Equation 2.27.
|
| Figure 2.6 Values of the thermodynamic parameter, Σ, for various saturated liquids as a function of the reduced temperature, T/TC. |
|
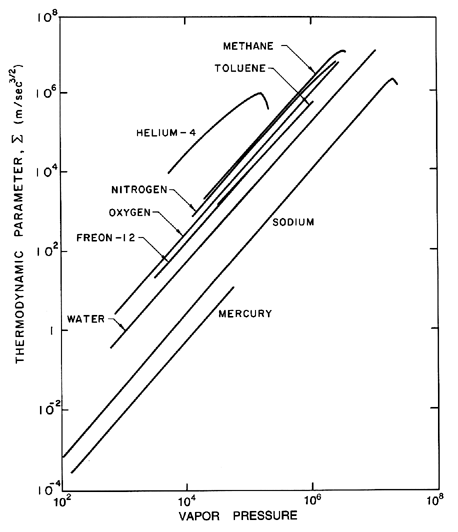
| Figure 2.7 Values of the thermodynamic parameter, Σ, for various saturated liquids as a function of the vapor pressure (in kg/m s2). |
First examine the case of bubble growth. The asymptotic growth rate given by Equation 2.33 is constant and hence in the characteristic case of a constant p∞, terms (1), (3), (4), (5), and (6) in Equation 2.12 are all either constant or diminishing in magnitude as time progresses. Furthermore, a constant, asymptotic growth rate corresponds to the case
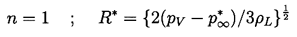 | ......(2.59) |
|---|
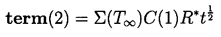 | ......(2.60) |
|---|
Using the relation 2.60, one can define a critical time, tc1 (called the first critical time), during the growth when the order of magnitude of term (2) becomes equal to the order of magnitude of the retained terms, as represented by (dR/dt)2. This first critical time is given by
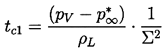 | ......(2.61) |
|---|
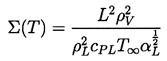 | ......(2.62) |
|---|
2.8 THERMALLY CONTROLLED GROWTH
When the first critical time is exceeded it is clear that the relative importance of the various terms in the Rayleigh-Plesset Equation, 2.12, will change. The most important terms become the driving term (1) and the thermal term (2) whose magnitude is much larger than that of the inertial terms (4). Hence if the tension (pV -p∞*) remains constant, then the solution using the form of Equation 2.24 for the thermal term must have n=½ and the asymptotic behavior is
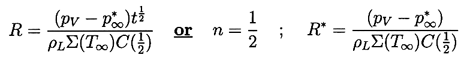 | ......(2.63) |
|---|
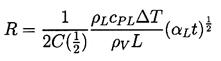 | ......(2.64) |
|---|
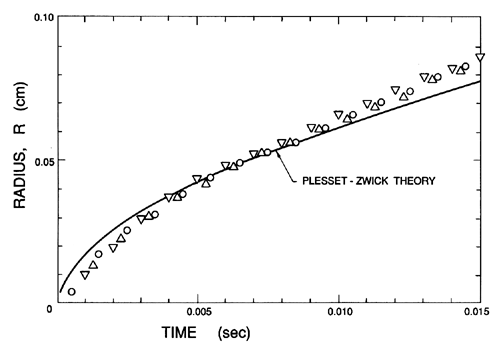
The result, Equation 2.63, demonstrates that the rate of growth of the bubble decreases substantially after the first critical time, tc1, is reached and that R subsequently increases like t½ instead of t. Moreover, since the thermal boundary layer also increases like (αLt)½, the Plesset-Zwick assumption remains valid indefinitely. An example of this thermally inhibited bubble growth is including in Figure 2.8, which is taken from Dergarabedian (1953). We observe that the experimental data and calculations using the Plesset-Zwick method agree quite well.
|
| Figure 2.8 Experimental observations of the growth of three vapor bubbles (three different symbols) in superheated water at 103.1°C compared with the growth expected using the Plesset-Zwick theory (adapted from Dergarabedian 1953). |
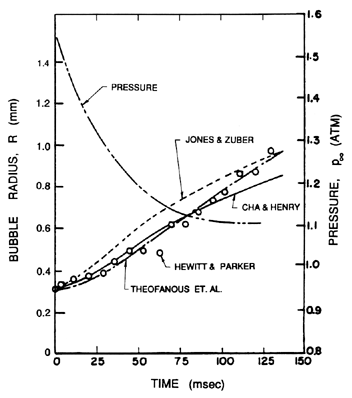
When bubble growth is caused by decompression so that p∞(t) changes substantially with time during growth, the simple approximate solution of Equation 2.63 no longer holds and the analysis of the unsteady thermal boundary layer surrounding the bubble becomes considerably more complex. One must then solve the diffusion Equation 2.15, the energy equation (usually in the approximate form of Equation 2.17) and the Rayleigh-Plesset Equation 2.12 simultaneously, though for the thermally controlled growth being considered here, most of the terms in Equation 2.12 become negligible so that the simplification, pV(TB)=p∞(t), is usually justified. When p∞ is a constant this reduces to the problem treated by Plesset and Zwick (1952) and later addressed by Forster and Zuber (1954) and Scriven (1959). Several different approximate solutions to the general problem of thermally controlled bubble growth during liquid decompression have been put forward by Theofanous et al. (1969), Jones and Zuber (1978) and Cha and Henry (1981). Theofanous et al. include nonequilibrium thermodynamic effects on which we comment in the following section. If these are ignored, then all three analyses yield qualitatively similar results which also agree quite well with the experimental data of Hewitt and Parker (1968) for bubble growth in liquid nitrogen. Figure 2.9 presents a typical example of the data of Hewitt and Parker and a comparison with the three analytical treatments mentioned above.
Several other factors can complicate and alter the dynamics of thermally controlled growth, and these are discussed in the sections which follow. Nonequilibrium effects are addressed in Section 2.9. More important are the modifications to the heat transfer mechanisms at the bubble surface that can be caused by surface instabilities or by convective heat transfer. These are reviewed in Sections 2.10 and 2.12.
One factor that could affect the dynamics of thermally controlled growth is whether or not the liquid at the interface is in thermal equilibrium with the vapor in the bubble. Most of the analyses assume that the temperature of the liquid at the interface, TLS, is the temperature of the saturated vapor in the bubble, TB . Theofanous et al. (1969) have suggested that this might not be the case because of the high evaporation rate. They employ an accommodation coefficient, Λ, defined (Schrage 1953) by
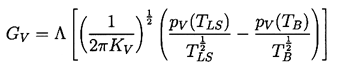 | ......(2.65) |
|---|
|
| Figure 2.9 Data from Hewitt and Parker (1968) on the growth of a vapor bubble in liquid nitrogen (pressure/time history also shown) and comparison with the analytical treatments by Theofanous et al. (1969), Jones and Zuber (1978), and Cha and Henry (1981). |
Theofanous et al. (1969) explore the effects of small values of Λ theoretically. They confirm that values of order unity do not yield bubble histories that differ by very much from those that assume equilibrium. Values of Λ of the order of 0.01 did produce substantial differences. However, the results using equilibrium appear to compare favorably with the experimental results as shown in Figure 2.9. This suggests that nonequilibrium effects have little effect on thermally controlled bubble growth though the issue is not entirely settled since some studies do suggest that values of Λ as low as 0.01 may be possible.
2.10 CONVECTIVE EFFECTS
Another way in which the rate of heat transfer to the interface may be changed is by convection caused by relative motion between the bubble and the liquid. Such enhancement of the heat transfer rate is normally represented by a Nusselt number, Nu, defined as the ratio of the actual heat transfer rate divided by the rate of heat transfer by conduction. Therefore in the present context the factor, Nu, should be included as a multiplier in the thermal term of the Rayleigh-Plesset equation. Then one seeks a relationship between Nu and the Peclet number, Pe=WR/αL, where W is the typical translational velocity of the bubble relative to the liquid. The appropriate relationship for a growing and translating bubble is not known; analytically this represents a problem that is substantially more complex than that tackled by Plesset and Zwick. Nevertheless, it is of interest to speculate on the form of Nu(Pe) and observe the consequences for the bubble growth rate. Therefore let us assume that this relationship takes the approximate form common in many convective heat transfer problems:
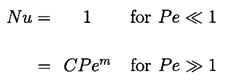 | ......(2.66) |
|---|
Despite the difficulties of accurate assessment of the convective heat transfer effects, let us consider the qualitative effects of two possible translational motions on a bubble growing like R=R*tn. The first effect is that due to buoyancy; the relative velocity, W, caused by buoyancy in the absence of viscous drag will be given approximately by gt. The viscous drag on the bubble will have little effect so long as νLt « R2. The second example is a bubble growing on a solid wall where the effective convective velocity is roughly given by dR/dt and hence W is proportional to R*tn-1. Thus the Peclet numbers for the two cases are respectively
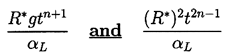 | ......(2.67) |
|---|
Consider first the case of inertially controlled growth for which n=1. Then it follows that convective heat transfer effects will only occur for Pe≥1 or for times t>tc2 where
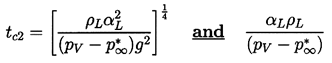 | ......(2.68) |
|---|
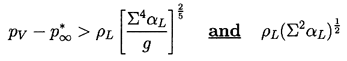 | ......(2.69) |
|---|
It follows that in each of the two bubble motions assumed there is some temperature below which one would expect Pe to reach unity prior to tc1. The question is what happens thereafter, for clearly the thermal effect that would otherwise begin at tc1 is now going to be altered by the enhanced heat transfer. When Pe>1 the thermal term in the Rayleigh-Plesset equation will no longer grow like t½ but will increase like t½/Nu which, according to the relations 2.67, is like t½-2m and t½-m for the two bubble motions. If, as in many convective heat transfer problems, m=½, it would follow that thermal inhibition of the growth would be eliminated and the inertially controlled growth would continue indefinitely.
Finally, consider the other possible scenario in which convective heat transfer effects might influence the thermally controlled growth in the event that tc2>tc1. Given n=½, the Peclet number for buoyancy-induced motion would become unity at
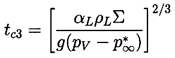 | ......(2.70) |
|---|
2.11 SURFACE ROUGHENING EFFECTS
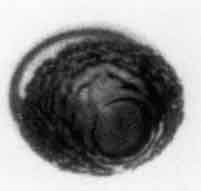
Another important phenomenon that can affect the heat transfer process at the interface during bubble growth (and therefore affect the bubble growth rate) is the development of an instability on the interface. If the bubble surface becomes rough and turbulent, the increase in the effective surface area and the unsteady motions of the liquid near that surface can lead to a substantial enhancement of the rate of heat transfer to the interface. The effect is to delay (perhaps even indefinitely) the point at which the rate of growth is altered by thermal effects. This is one possible explanation for the phenomenon of vapor explosions which are essentially the result of an extended period of inertially controlled bubble growth.
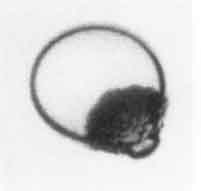
| 
| 
|
Shepherd and Sturtevant (1982) and Frost and Sturtevant (1986) have examined rapidly growing nucleation bubbles near the limit of superheat and have found growth rates substantially larger than expected when the bubble was in the thermally controlled growth phase. The experiments examined bubble growth within droplets of superheated liquid suspended in another immiscible liquid. Typical photographs are shown in Figure 2.10 and reveal that the surfaces of the bubbles are rough and irregular. The enhancement of the heat transfer caused by this roughening is probably responsible for the larger growth rates. Shepherd and Sturtevant (1982) attribute the roughness to the development of a baroclinic interfacial instability similar to the Landau-Darrieus instability of flame fronts. It is also of interest to note that Frost and Sturtevant report that the instability could be suppressed by increasing the ambient pressure and therefore the temperature and density within the bubble. At an ambient pressure of 2bar, the onset of the instability could be observed on the surface of ether bubbles and was accompanied by a jump in the radiated pressure associated with the sudden acceleration in the growth rate. At higher ambient pressures the instability could be completely suppressed. This occurs because the growth rate of the instability increases with the rate of growth of the bubble, and both are significantly reduced at the higher ambient pressures. It may be that, under other circumstances, the Rayleigh-Taylor instabilities described in Section 2.12 could give rise to a similar effect.
2.12 NONSPHERICAL PERTURBATIONS
Apart from the phenomena described in the preceding section, it has, thus far, been tacitly assumed that the bubble remains spherical during the growth or collapse process; in other words, it has been assumed that the bubble is stable to nonspherical distortions. There are, however, circumstances in which this is not true, and the subsequent departure from a smooth spherical shape can have important practical consequences.
The stability to nonspherical disturbances has been investigated from a purely hydrodynamic point of view by Birkhoff (1954) and Plesset and Mitchell (1956), among others. These analyses essentially examine the spherical equivalent of the Rayleigh-Taylor instability; they do not include thermal effects. If the inertia of the gas in the bubble is assumed to be negligible, then the amplitude, a(t), of a spherical harmonic distortion of order n (n>1) will be governed by the equation:
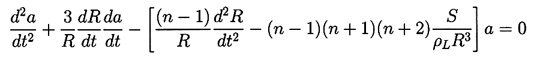 | ......(2.71) |
|---|
The fact that the coefficients in Equation 2.71 are not constant in time causes departure from the equivalent Rayleigh-Taylor instability for a plane boundary. The coefficient of a is not greatly dissimilar from the case of the plane boundary in the sense that instability is promoted when d2R/dt2>0 and surface tension has a stabilizing effect. The primary difference is caused by the da/dt term, which can be interpreted as a geometric effect. As the bubble grows the wavelength on the surface increases, and hence the growth of the wave amplitude is lessened. The reverse occurs during collapse.
Plesset and Mitchell (1956) examined the particular case of a vapor/gas bubble initially in equilibrium that is subjected to a step function change in the pressure at infinity. Thermal and viscous effects are assumed to be negligible. The effect of a fixed mass of gas in the bubble will be included in this presentation though it was omitted by Plesset and Mitchell. Note that this simple growth problem for a spherical bubble was solved for R(t) in Section 2.4. One feature of that solution that is important in this context is that d2R/dt2≥0. It is this feature that gives rise to the instability. However, in any real scenario, the initial acceleration phase for which d2R/dt2≥0 is of limited duration, so the issue will be whether or not the instability has sufficient time during the acceleration phase for significant growth to occur.
It transpires that it is more convenient to rewrite Equation 2.71 using y=R/Ro as the independent variable rather than t. Then a(y) must satisfy
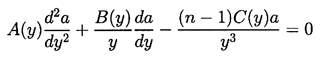 | ......(2.72) |
|---|
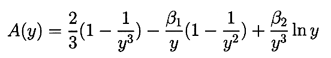 | ......(2.73) |
|---|
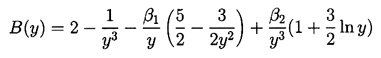 | ......(2.74) |
|---|
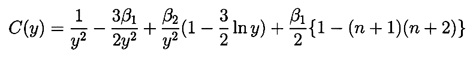 | ......(2.75) |
|---|
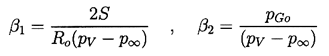 | ......(2.76) |
|---|
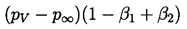 | ......(2.77) |
|---|
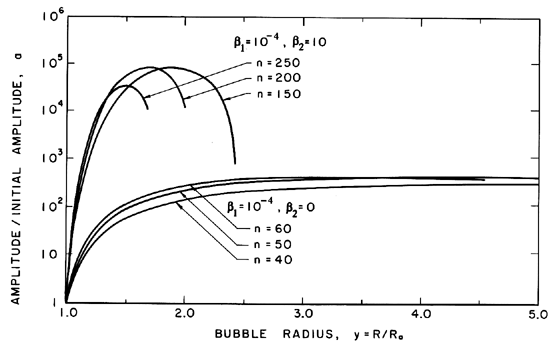
|
| Figure 2.11 Examples of the growth of the amplitude, a, of a spherically harmonic disturbance (of order n as indicated) on the surface of a growing cavitation bubble for two typical choices of the surface tension and gas content parameters, β1 and β2. |
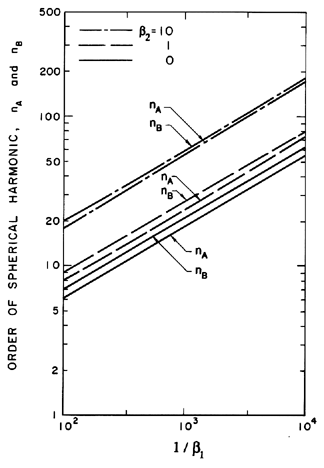
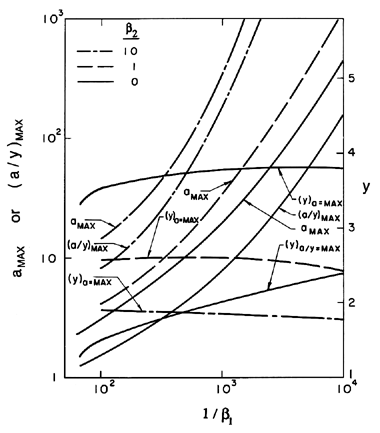
Some typical numerical integrations of Equation 2.72 in cases of bubble growth are shown in Figure 2.11 where the amplitude scale is arbitrary. Plesset and Mitchell performed hand calculations for small n and found only minor amplification during growth. However, as can be anticipated from Equation 2.72, the amplitudes may be much larger for large n. It can be seen from Figure 2.11 that the amplitude of the disturbance reaches a peak and then decays during growth. For given values of the parameters β1 and β2, there exists a particular spherical harmonic, n=nA, which achieves the maximum amplitude, a; in Figure 2.11 we chose to display data for n values which bracket nA. The dependence of nA on β1 and β2 is shown in Figure 2.12. A slightly different value of n denoted by nB gives the maximum value of a/y. Since the latter quantity rather than a represents a measure of the wave amplitude to wavelength ratio, Figure 2.12 also shows the dependence of nB on β1 and β2. To complete the picture, Figure 2.13 presents values of (a)max, (a/y)max and the sizes of the bubble (y)a=max and (y)a/y=max at which these maxima occur.
|
| Figure 2.12 The orders of the spherical harmonic disturbances that, during bubble growth, produce (i) the maximum disturbance amplitude (nA) and (ii) the maximum ratio of disturbance amplitude to bubble radius (nB) for various surface tension and gas content parameters, β1 and β2. |
|
| Figure 2.13 The maximum amplification, amax, and the maximum ratio of amplitude to bubble radius, (a/y)max, for spherical harmonic disturbances on the surface of a growing bubble for various surface tension and gas content parameters, β1 and β2. Also shown are the bubble sizes, (y)a=max and (y)a/y=max, at which these maxima occur. |
In summary, it can be seen that the initial acceleration phase of bubble growth in which d2R/dt2≥0 is unstable to spherical harmonic perturbations of fairly high order, n. On the other hand, visual inspection of Equation 2.71 is sufficient to conclude that the remainder of the growth phase during which dR/dt>0, d2R/dt2<0 is stable to all spherical harmonic perturbations. So, if inadequate time is available for growth of the perturbations during the acceleration phase, then the bubble will remain unperturbed throughout its growth. In their experiments on underwater explosions, Reynolds and Berthoud (1981) observed bubble surface instabilities during the acceleration phase that did correspond to fairly large n of the order of 10. They also evaluate the duration of the acceleration phase in their experiments and demonstrate, using an estimated growth rate, that this phase is long enough for significant roughening of the surface to occur. However, their bubbles become smooth again in the second, deceleration phase of growth. The bubbles examined by Reynolds and Berthoud were fairly large, 2.5cm to 4.5cm in radius. A similar acceleration phase instability has not, to the author's knowledge, been reported for the smaller bubbles typical of most cavitation experiments. This could either be the result of a briefer acceleration phase or the greater stabilizing effect of surface tension in smaller bubbles.
- Birkhoff, G. (1954). Note on Taylor instability. Quart. Appl. Math., 12, 306--309.
- Blake, F.G. (1949). The onset of cavitation in liquids: I. Acoustics Res. Lab., Harvard Univ., Tech. Memo. No. 12.
- Ceccio, S.L. and Brennen, C.E. (1991). Observations of the dynamics and acoustics of travelling bubble cavitation. J. Fluid Mech., 233, 633--660.
- Cha, Y.S. and Henry, R.E. (1981). Bubble growth during decompression of a liquid. ASME J. Heat Transfer, 103, 56--60.
- Daily, J.W. and Johnson, V.E., Jr. (1956). Turbulence and boundary layer effects on cavitation inception from gas nuclei. Trans. ASME, 78, 1695--1706.
- Dergarabedian, P. (1953). The rate of growth of vapor bubbles in superheated water. ASME J. Appl. Mech., 20, 537--545.
- Epstein, P.S. and Plesset, M.S. (1950). On the stability of gas bubbles in liquid-gas solutions. J. Chem. Phys., 18, 1505--1509.
- Forster, H.K. and Zuber, N. (1954). Growth of a vapor bubble in a superheated liquid. J. Appl. Phys., 25, No.4, 474--478.
- Frost, D. and Sturtevant, B. (1986). Effects of ambient pressure on the instability of a liquid boiling explosively at the superheat limit. ASME J. Heat Transfer, 108, 418--424.
- Fujikawa, S. and Akamatsu, T. (1980). Effects of the non-equilibrium condensation of vapour on the pressure wave produced by the collapse of a bubble in a liquid. J. Fluid Mech., 97, 481--512.
- Hewitt, H.C. and Parker, J.D. (1968). Bubble growth and collapse in liquid nitrogen. ASME J. Heat Transfer, 90, 22--26.
- Jones, O.C. and Zuber, N. (1978). Bubble growth in variable pressure fields. ASME J. Heat Transfer, 100, 453--459.
- Neppiras, E.A. and Noltingk, B.E. (1951). Cavitation produced by ultrasonics: theoretical conditions for the onset of cavitation. Proc. Phys. Soc., London, 64B, 1032--1038.
- Noltingk, B.E. and Neppiras, E.A. (1950). Cavitation produced by ultrasonics. Proc. Phys. Soc., London, 63B, 674--685.
- Plesset, M.S. (1949). The dynamics of cavitation bubbles. ASME J. Appl. Mech., 16, 228--231.
- Plesset, M.S. and Zwick, S.A. (1952). A nonsteady heat diffusion problem with spherical symmetry. J. Appl. Phys., 23, No. 1, 95--98.
- Plesset, M.S. and Mitchell, T.P. (1956). On the stability of the spherical shape of a vapor cavity in a liquid. Quart. Appl. Math., 13, No. 4, 419--430.
- Plesset, M.S. and Prosperetti, A. (1977). Bubble dynamics and cavitation. Ann. Rev. Fluid Mech., 9, 145--185.
- Poritsky, H. (1952). The collapse or growth of a spherical bubble or cavity in a viscous fluid. Proc. First Nat. Cong. in Appl. Math., 813--821.
- Rayleigh, Lord. (1917). On the pressure developed in a liquid during the collapse of a spherical cavity. Phil. Mag., 34, 94--98.
- Reynolds, A.B. and Berthoud, G. (1981). Analysis of EXCOBULLE two-phase expansion tests. Nucl. Eng. and Design, 67, 83--100.
- Schrage, R.W. (1953). A theoretical study of interphase mass transfer. Columbia Univ. Press.
- Scriven, L.E. (1959). On the dynamics of phase growth. Chem. Eng. Sci., 10, 1--13.
- Shepherd, J.E. and Sturtevant, B. (1982). Rapid evaporation near the superheat limit. J. Fluid Mech., 121, 379--402.
- Theofanous, T., Biasi, L., Isbin, H.S., and Fauske, H. (1969). A theoretical study on bubble growth in constant and time-dependent pressure fields. Chem. Eng. Sci., 24, 885--897.
Last updated 12/1/00.
Christopher E. Brennen